Research and Development
&
Technology
HYALURONIC ACID – Highly advanced technology and manufacturing process permits Xcelens to gain a complete and pure Hyaluronic Acid (HA) with the parameters specially “tailored” for producing injectable hydrogel products.
Hyaluronic acid, which is used by Xcelens, can be safely called one of the most purified hyaluronic acid on the market.
Since it is stable and hypoallergenic, streptococcus bacteria is used. However, residues of protein molecules may remain in the final product, which can produce undesirable phenomena in the form of delayed-type hyperergic reactions.
Therefore, Xcelens, maintaining the image of the Swiss brand fillers, uses innovative filtration systems to create highly purified and safe hyaluronic acid of pharmacopoeial purity class.
Technology
The entire Genyal range benefits from a very advanced purification and homogenisation process:
- Pure hyaluronic acid derived from Bio-fermentation, this 100% Non-animal sourced. After having aligned the hyaluronic chains, the next step is cross-linking with BDDE. Creating, using the latest innovations, a three-dimensional matrix with a very fine mesh, making it possible to obtain a more homogeneous gel that other formulas.
- Immediately after this, a new, exclusive stage of homogenisation and optimisation of the gel’s visco-elasticity is used to make the injection fluid and regular, so the product will be highly viscous and elastic.
- All this makes it possible for a very dense gel to pass through a 27G needle, with spectacular cosmetic effects and exceptional duration!
- Thus, through a highly sophisticated purification procedure, all the undesired chemical components, including oxygen, are eliminated, allowing the product to reach full stability in the syringe.
- The protein residue in the Genyal range, partly because of the continual monitoring of the purification and sterilisation phases throughout the production process, is one of the lowest of the fillers on the market; furthermore, the level of endotoxin (< 0.05 EU/g) is substantially lower than European Pharmacopoeia parametrs.

Phase 1

Phase 2

Phase 3

Phase 4

Phase 5
Phase 6
Result
As result it meets all european quality standards (es iso10993) and has the following qualities:
Natural correction with a high plasticity and biocompatibility, effective correction of the tissue even if dynamically active zones without hypercorrection and long lasting result. All this makes it possible for a very dense gel to pass through a 27G needle, with spectacular cosmetic effects and exceptional duration. A highly sophisticated purification procedure, all the undesired chemical components, including oxygen, are eliminated, allowing the product to reach full stability in the syringe.
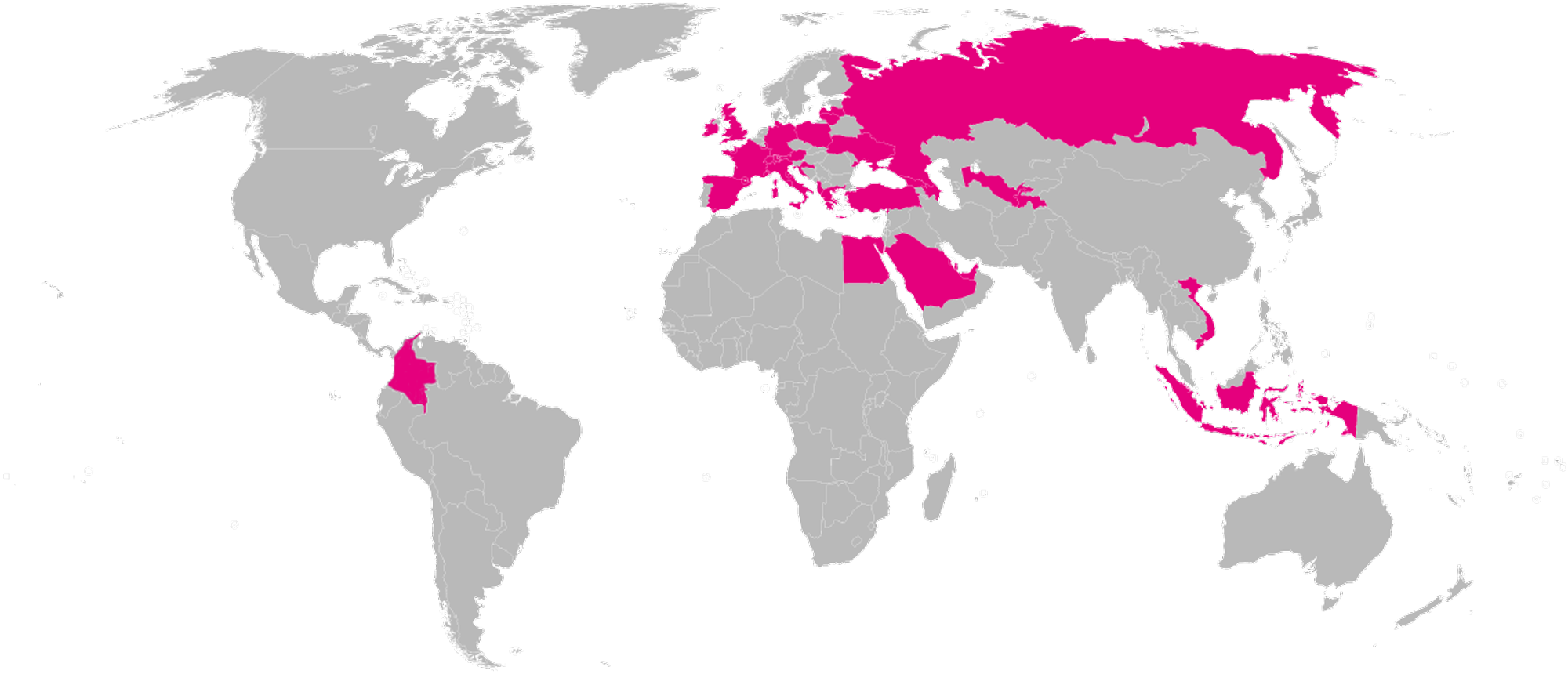
Distributors
Here is a map with our distributor countries.